Color Theory Hydrogen
| Grey | Blue | Green |
| Established process for hydrogen production. Both the energy required and the starting materials come from fossil fuels such as coal or natural gas. At temperatures of 700 to 1,000 °C and with the help of steam, hydrogen is extracted from a methane source and enriched in further reaction steps. The process is efficient and tried and tested. And unfortunately harmful to the climate, as every kilogram of gray hydrogen is “paid for” with seven kilograms of CO2. 95 percent of the hydrogen produced today is gray. | Production of gray hydrogen. The raw materials here are also fossil fuels, from which methane vapor is extracted and then hydrogen is separated. However, the CO2 released is captured and either stored (injection into underground sandstone layers) or reused in the chemical industry. | Production of hydrogen by electrolysis. Regeneratively generated energy is used to split water into its components, oxygen and hydrogen. No CO2 is released. However, there is still room for improvement in the energy balance, which is why only a fraction of hydrogen has been produced green to date. |
The AEM electrolysis process
In the production of green hydrogen, electricity generated from renewable sources is used in direct current format. The two electrodes are located in a container, the so-called cell. The anode and cathode are separated by a partially permeable membrane. An alkaline solution or acid serves as the electrolyte. If an electric current is now applied to the electrodes (cathode, negative pole and anode, positive pole), a chemical reaction takes place at both electrodes. Hydrogen is released at the cathode and oxygen is released at the anode. A single hydrogen atom and a residual molecule OH- are formed. The extremely reactive single hydrogen atom immediately combines with another hydrogen atom and is then transported away via a gas diffusion layer and collected.
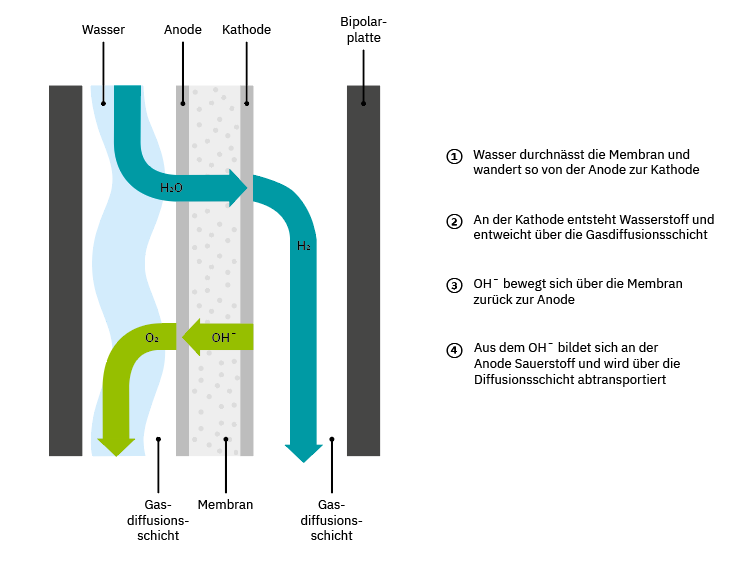
The cell is divided by a membrane to keep the two parts of the split water molecule reliably separated. When they come together, they immediately join again. And they do so with momentum. If this reunion is uncontrolled, it is called an oxyhydrogen reaction.






In the process used by Enapter, only a highly diluted potassium hydroxide solution is used instead of the usual strong acids. These require PCB and titanium for the container and electrodes in order to withstand the aggressive substance in the long term. With the AEM electrolyzer, steel and non-precious metals are sufficient. This makes the electrolyzer significantly cheaper and easier to handle. Thanks to sophisticated pressurization between the half cells, Enapter achieves a high degree of purity of the hydrogen obtained.



